Abstract
Introduction
Dedicator of Cytokinesis 1 (DOCK1), a unique member of guanine nucleotide exchange factors, was discovered to modulate the metastasis and survival in breast cancer and glioblastoma. As a novel Rac activator, DOCK1 was critical for oncogenic Ras-induced cellular migration and invasion. However, the expression and functional role of DOCK1 in acute myeloid leukemia (AML) have not been elucidated to date. We hypothesized that DOCK1 contributed to leukemogenesis and predicted inferior outcome in patients with AML. The aim of this study is to determine the prognostic significance of DOCK1 in the context of established predictive molecular markers and derive DOCK1 -associated gene and microRNA (miRNA) expression signatures in AML patients.
Methods
Multiple cohorts of de novo AML patients were enrolled in the present study. Bone marrow (BM) samples (MILE research program-stage I) and CD34+ cells (de Jonge et al ., 2011) from AML patients and healthy donors were evaluated for DOCK1 expression. Patients treated with protocols of Dutch-Belgian Cooperative Trial Group for Hematology Oncology (HOVON, Verhaak et al ., 2009), from AMLCG-1999 trial and National Taiwan University Hospital (NTUH) were included to investigate the prognostic significance of DOCK1 in AML. Gene and miRNA expression profiles from The Cancer Genome Atlas (TCGA) were obtained to study DOCK1-associated genomic signatures in AML. Kaplan-Meier survival curves analysis with log-rank test, univariate and multivariate Cox regression model analyses of overall survival (OS) and event-free survival (EFS) were conducted. Functional enrichment analyses of gene ontology (GO), kyoto encyclopedia of genes and genomes (KEGG) and gene set enrichment analysis (GSEA) in gene expression profiles were performed.
Results
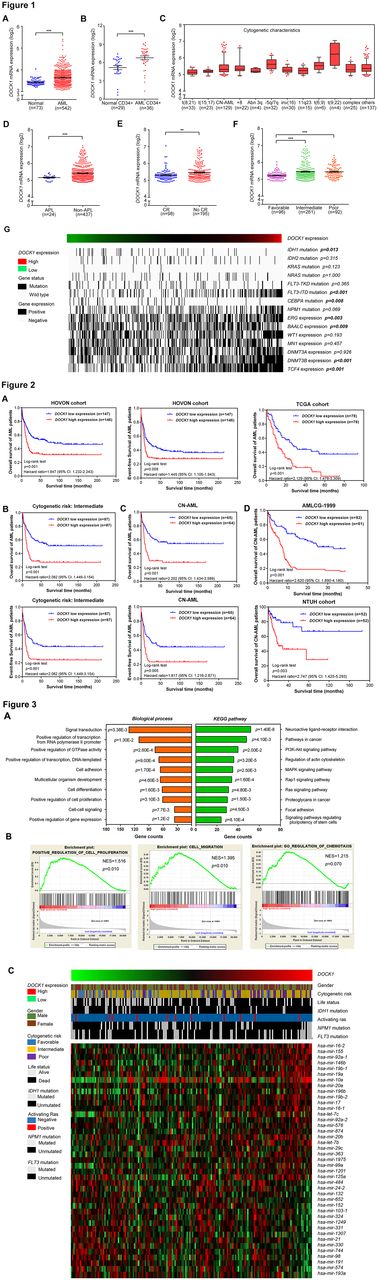
In the present study, we observed aberrantly elevated level of DOCK1 expression in BM samples and CD34+ cells of AML patients compared with healthy donors group (Figure 1A-B). DOCK1 expression pattern of cytogenetic features was characterized in patients from HOVON cohort, and higher expression level in unfavorable prognostic category was identified (Figure 1C). The patients were further stratified by APL subtype, CR rate and NCCN cytogenetic risk. Significantly increased level of DOCK1 was detected in non-APL or intermediate/ poor risk patients with lower CR rate (Figure 1D-F). Overexpression of DOCK1 was implicated in significantly association with mutated IDH1 , FLT3-ITD , CEBPA and positive expression of ERG , BAALC , DNMT3B and TCF4 (Figure 1G).
DOCK1 high expression was observed in significant association with reduced OS and EFS of AML patients from the HOVON cohort, which was further validated in OS of the TCGA cohort (Figure 2A). Besides, within the subgroups by cytogenetic risk, high expression of DOCK1 predicted significant adverse outcome in OS and EFS of intermediate-risk cases (Figure 2B). In addition, CN-AML patients with DOCK1 high expression showed a significant decreased OS and EFS (Figure 2C), which were confirmed in patients from AMLCG-1999 trial and Asian patients (Figure 2D). Moreover, multivariate survival analyses demonstrated the independent prognostic significance of DOCK1 in OS ( p =0.017) after adjustment for age, CEBPA mutation, FLT3-ITD mutation and DNMT3B expression.
To gain insight into leukemogenic mechanisms associated with DOCK1 expression, genome-wide gene and miRNA expression signatures were analyzed. 2615 differentially expressed genes were identified for bioinformatics analysis. Annotations of functional enrichment analyses indicated that DOCK1 potentially regulated cell proliferation, migration and oncogenic pathways in AML (Figure 4A-B). Moreover, a heatmap was plotted and depicted DOCK1 -associated miRNA signatures. Among them, miR155, let-7b, miR16-1, miR21 and miR24-2 have been identified in leukemias, suggesting the modulated networks of DOCK1 in AML (Figure 4C).
Conclusion
Our study provides evidence for the first time that DOCK1 high expression independently predict poor outcome in AML patients, particularly among intermediate risk cytogenetic subgroups and CN-AML patients. Unique gene and miRNA signatures associated with DOCK1 expression presented distinct features in leukemogenesis. DOCK1 was demonstrated as a robust biomarker to complement risk assessment in AML, highlighting novel therapeutic strategy for anti-leukemic interventions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal